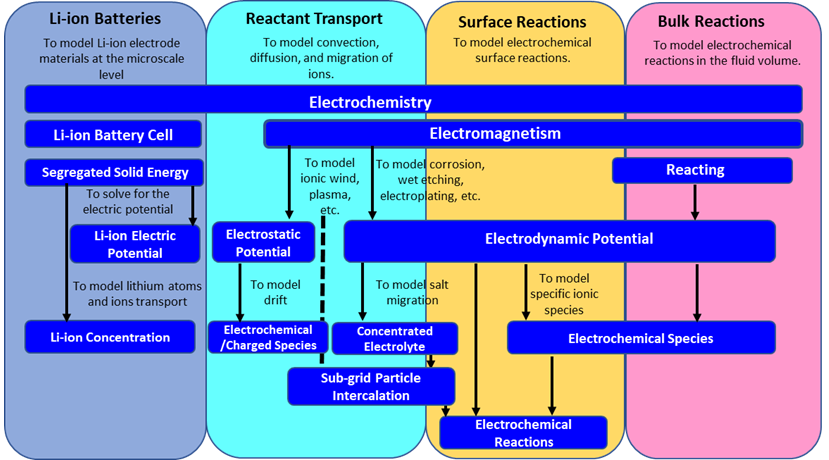
Electrochemistry
Electrochemistry is the study of chemical reactions which occur due to an imposed electrical charge, or a difference in electrical potential at a boundary between a conductor–such as a metal–and an electrolyte. Simcenter STAR-CCM+ provides models that allow you to simulate batteries, corrosion, etching, and other electrochemical reactions.
Typically, during electrochemical reactions, electrons are produced or consumed at the boundary between a metal and an electrolyte. Metal and hydrogen atoms can lose electrons to become positive ions, and non-metal atoms can gain electrons to become negative ions. Both negative and positive ions are free to move through an electrolyte solution, however, electrons cannot move through an electrolyte solution—only through a conductor such as a metal. The rate of electrochemical reactions and the transport of ions is directly related to the magnitude of the electrical charge that is imposed, or the difference in electric potential across the boundary.
You can use Simcenter STAR-CCM+ to model a wide variety of electrochemical applications and simulate behavior such as, movement of ions or specific ionic species, ionic charge density, and accumulation of product species.
Simcenter STAR-CCM+ supports the following general categories of electrochemical modeling:
- Li-Ion Battery Cells
- Ionic Species Flux
- Electrochemical Surface Reactions
- Bulk Ion Chemical Reactions
You can simulate electrochemical surface reactions and surface chemistry reactions within the same region, using both the Electrochemical Reactions model and the Surface Chemistry model. See Surface Chemistry Workflow.
The following diagram shows an overview of these general workflows with examples of some common areas of electrochemistry which relate to each workflow, and the corresponding electrochemistry models that are required in Simcenter STAR-CCM+.