There are many applications that involve the transport of reacting
particles, such as calcination and char oxidation. You can simulate the process of
interphase reactions using Lagrangian Multiphase, Multiphase or Fluid Film models.
The following diagram explains the difference between interphase and intraphase reactions between species.
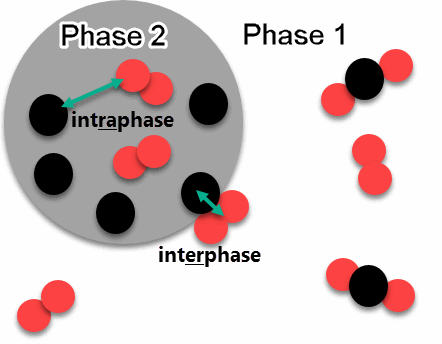
- Intraphase reactions occur between atoms/molecules in the same phase.
- Interphase reactions occur between atoms/molecules in different phases at the interface between the different phases.
Lagrangian Multiphase Interphase Reactions
You can simulate the process of reacting particles (liquid or solid) using one of the Lagrangian Multiphase models. The particles (liquid or solid) can interact with the continuous phase using the Two-Way Coupling model.
When simulating applications such as coal or biomass combustors, you can use the Lagrangian Particles model to simulate small solid particles that are traveling in a flow. Larger clumps are simulated as Discreet Element Method (DEM) particles.
Simcenter STAR-CCM+ also provides the Particle Porosity model which you can use to specify a factor by which solid particles shrink due to reactions.
Particle components can devolatilize or react with a material in a gas phase to form products in gas, liquid, or solid phases.
To model the transport of reacting particles, the Material Particles model is required in the Lagrangian phase. Material particles have mass and volume, obey the physical conservation laws of mass and momentum, and are assumed to be internally homogenous, without internal motion. The type of Material model that is selected influences the type of mass transfer model that is made available:
- When Multi-Component Particle is selected, you can select
Mass Transfer.
You can then model the following types of particle reaction (use these models together or separately):
- Particle Reaction
- Particle Devolatilization
- When Multi-Component Coal is selected, you can select
Coal Combustion. You can use the Coal Combustion model to simulate coal furnaces and gasification.
- Particle Reaction
-
- You can use the Particle Reaction model to simulate various particle reactions such as char oxidation, biomass particle oxidation, and ferric oxide reduction. It is possible to simulate multiple reactions between solid particles or liquid droplets reacting with a gas-phase or liquid-phase species to form solid, liquid, and/or gas-phase products.
- Particle Devolatilization
-
- The Particle Devolatilization model can simulate various particle mass transfer processes, such as gasification, sublimation, phase transformation, and biomass/coal devolatilization. Particle devolatilization is a gasification process where one or more solid or liquid particles are converted to volatile matter and/or a residue.
- Coal Combustion
-
- Coal is an important fuel that is used in electricity generation worldwide. It is pulverized to a fine powder before being blown into the combustion chambers of high-temperature furnaces.
- The Coal Combustion model in
Simcenter STAR-CCM+ has pre-defined species and reactions that are designed specifically for simulating coal combustion. You can use this model to simulate the transport, gasification, and combustion of coal particles as they pass through such furnaces. This model also allows you to simulate coal fuel NOx emissions.
- Coal is a composite material consisting of organic and inorganic substances that can also be moist. The coal particle is assumed to be initially coated with moisture so that a single-component evaporation treatment is appropriate. The driving force for evaporation of this layer is the departure from equilibrium of the liquid-vapor system. In coal combustion, the moisture evaporation is usually heat-transfer limited and takes place rapidly. The devolatilization and char oxidation reactions occur after the moisture has evaporated. In addition to an appropriate combustion model in the continuous phase, coal combustion requires the following Lagrangian phase submodels:
- Coal Moisture Evaporation
Represents the Quasi-Steady Evaporation model for the moisture component (H2O) of the coal particle. This model allows the H2O component of the coal particle to lose its mass through a quasi-steady evaporation process.
- Raw Coal Devolatilization
Defines how the combustible substances are made available for combustion, using either the Two-Step Devolatilization model or the User-Defined Devolatilization model. The Volatile Vapor Component property allows you to select the species to which the raw coal devolatilizes. If no species is selected, an error message (Error: no CoalVolatile vapor selected for Devolatilization model in coal) is issued when the solution field is initialized. The benefit of this property is that it allows you to model two different coal types with two different types of CoalVolatiles.
- Two-Step Devolatilization
Controls the process by which raw coal is converted to gaseous combustible material (volatile matter) and solid char residue. Experimental studies have shown that, under certain heating conditions, the volatile matter yield is higher than the value that is obtained from standard proximate analysis. This effect is accounted for by allowing the coal material to devolatilize by two temperature-dependent competing reactions—the Two-Step Devolatilization model
[751].
- User-Defined Devolatilization
This model allows you to define the devolatilization rate for the raw coal component of the coal. In addition, you can set the volatile yield in the properties of this model.
- Char Oxidation:
There are three types of char oxidation models available. Each specific char oxidation model node contains three reaction nodes, one for each of the possible char oxidation processes.
- Char Reaction With O2
- Char Reaction With H2O
- Char Reaction With CO2
Only the first reaction, Char Reaction With O2, is active by default. If you require a more accurate simulation and have reaction rates for the other two reactions, you can activate the two further optional reactions.
- First-Order Char Oxidation
This model is used to predict the rate at which the char content of the coal burns. The time that is required for this char burnout is a significant part of the coal combustion process. The First-Order Char Oxidation model consists of one or more First-Order Combined Rate reactions that account for gas phase diffusion of the oxidizer as well as the first-order surface reaction kinetics.
- Half-Order Char Oxidation
The Half-Order Char Oxidation model consists of one or more Half-Order Combined Rate reactions that account for gas phase diffusion of the oxidizer as well as the half-order surface reaction kinetics.
- User-Defined Char Oxidation
This model provides an alternative to the two other char oxidation rates which you can use to set custom rates as a profile or as a constant.
- When these Lagrangian phase models are used with a combustion model in the continuous phase—and if the Two-Way Coupling phase model is also active—the complete coal combustion process can be realized. It is also possible to model a non-reacting Lagrangian phase containing the multi-component coal material model only, without activating coal combustion.
- Particle Porosity
- Simcenter STAR-CCM+ also provides the optional Lagrangian model,
Particle Porosity, which allows you to define how the porosity of particles which burn internally changes through the course of a coal combustion reaction. The porosity of a particle determines how the diameter and surface area of the particle changes throughout a reaction. When the surface area of a particle changes, the overall reaction rate is affected.
Multiphase Interphase
Reactions
You can simulate reactions between two
different Eulerian phases using the Interphase Reaction model. For example, between
fluid and solid particles in a fluidized bed reactor.
- Interphase Reaction
- The Interphase Reaction model
is available to use with the Eulerian Multiphase (EMP) , Volume of Fluid
(VOF), or Mixture Multiphase (MMP) variants of the Multiphase model.
- The
Interphase Reaction model allows you to specify the reactant and product
species from either of the phases that are defined within a phase
interaction. Phase interactions can define reactions between two phases
of the same or different material types—Multi-component Gas,
Multi-component Liquid, or Multi-component Particle. You can define
specific species which are involved in the interphase reaction from
either phase and also specify to which phase each product belongs.
Fluid Film Interphase Reactions
You can simulate reactions between a fluid film phase and its neighbouring gas phase using the Interphase Reaction model in conjunction with the Fluid Film model.
Similar to Multiphase Interphase Reactions, the
Interphase Reaction model for fluid film allows you to model reactions between two
phases, where the fluid film phase is defined as a multi-component liquid and the
gas phase is defined as a multi-component gas.
