Chemical Kinetics
Chemical kinetics is concerned with the mechanisms and rates of reaction of chemical species at the molecular level.
When modeling reacting species transport, providing a source term to the transport equations requires calculation of the reaction rates (chemical kinetics) for all reactions in the chemical mechanism.
Reaction Rate
The reaction rate for elementary reactions is calculated as follows.
there are two reactant species and one product species. In order for the reaction to proceed, the reactant molecules must collide with energy that is equal to or greater than the activation energy . The rate at which this overall reaction proceeds is also dependant upon the rate constant , and for an elementary reaction, the concentration of the two reactants to the power of their stoichiometric coefficients.
The chemical formula of a species in square brackets represents the concentration of that species in the reaction mixture.
The production (or consumption) rate of species , depends on the reaction rates of the reactions which produce and consume the species, and on the concentration of all species which participate in those reactions. The total net rate is therefore a sum of Eqn. (3360) over all reactions in which species participates. In the equation below, (=1, 2, … , ) denotes reactions and denotes species.
The net reaction rate of species i, denoted , is the sum over all reactions ( in Eqn. (3358)) multiplied by the stoichiometric coefficient.
where the rate coefficients, for the forwards reaction and for the backwards reaction, are determined by Eqn. (3365).
The user-defined reaction rate is calculated from:
Rate Constant
- the temperature exponent
- the activation energy
- the frequency factor, or pre-exponential factor
Chemical Heat Release Rate
The chemical heat release rate is defined as:
where is the net reaction rate of the species, and is the formation enthalpy of the species. When using the Species Transport models, Eqn. (3366) is evaluated and available as a field function named Chemical Heat Release Rate.
For the Flamelet Generated Manifold (FGM), and partially premixed Chemical Equilibrium (CE) and Steady Laminar Flamelet (SLF) models, the chemical heat release rate is calculated as:
where is the progress variable source term, and is the heat of reaction. is calculated assuming the fuel is a hydro-carbon reacting with oxygen in a 1-step reaction as:
The heat of reaction is calculated as:
where is the mass fraction of the species in the fuel stream.
For non-premixed SLF, the chemical heat release rate is calculated as:
where is the scalar dissipation rate.
Note that the chemical heat release rates for flamelet models are an approximation of the exact heat release rate from Eqn. (3366), and the field function is named Chemical Heat Release Rate Indicator.
Third Body Reactions
- Third Body Efficiencies
- Some reactions require a “third body” in order to proceed, as in: (3371)where represents the third body. Third bodies act as a form of catalyst. Typically, any species in the mixture can act as a third body. Third bodies affect the rate at which the reaction proceeds.
- Pressure-Dependent Chemical Reaction Rates
- Pressure dependent chemical reactions (fall-off reactions) have a first-order rate at low pressure and become zero-order as the concentration of the third-body species increases. In this type of reaction, the rate is affected by pressure as well as temperature,
Simcenter STAR-CCM+ calculates the rate constants using the pre-exponential factor
that is provided by the reaction mechanism: (3375)
Chemically-Activated Bimolecular Reaction Rates
Pressure Dependence Through Logarithmic Interpolation (PLOG)
When using the PLOG method to describe the pressure dependence of a reaction rate, the reaction rate is calculated by the Arrhenius expression Eqn. (3365) using rate parameters ( , , and ) that are modified for different pressures.
Rate parameters are given for a discrete set of (at least two) different pressures within the pressure range of interest.
- When the pressure
in a simulation is between given pressures (for example, between
and
), the rate parameter is calculated by a linear interpolation of
as a function of
:(3384)
- When the pressure within a simulation is higher or lower than any of the given pressures, the rate parameters for the highest or lowest pressures, respectively, are used.
Chemical Equilibrium
When the forwards and backwards reaction rates are equal, the reaction is said to have reached equilibrium and no net reaction is observed. The proportions of reactants and products are constant as long as the pressure (for gas-phase reactions), temperature, and concentrations of species are constant.
You can determine the amount of each species that is present at equilibrium using the equilibrium constant, .
where is a reference concentration.
Surface Chemistry
- Gas-phase molecules colliding with surface molecules and becoming adsorbed. The surface site (the reactive molecules of the surface) becomes part of the bulk and the previous gas-phase molecules form the new surface on top of the bulk.
- Surface reactions between molecules on the surface, or one molecule on the surface and one in the gas, reacting to form new surface, gas phase, or bulk molecules.
- Desorption of a surface molecule, caused by the molecule entering the gas phase after a reaction, or simply by overcoming the bonding energy.
For example, in the diagram below, a gas molecule is adsorbed onto a surface next to a previously adsorbed surface species. The gas molecule is broken up into its constituent atoms. Some of the adsorbed atoms react to form smaller gas molecules which leave the surface. One atom from the gas molecule remains adsorbed as a surface species, and the previously adsorbed surface species becomes a bulk species.
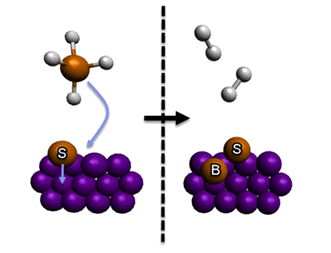
The reaction in the diagram above can be represented by:
Bulk species are not considered reactive but can end up on a surface site if the surface molecules previously covering the bulk species are desorbed or take part in a reaction.
- Species in the gas phase,
- Species on the surface at the gas-surface interface, . Surface reactions can be described as using either open site formalism or atomic site formalism.
- Species in the solid layer—bulk species, .
The general surface reaction form for reaction is:
where:
- , , and are gas, site, and bulk species.
- , , and are the numbers of gas, site, and bulk species.
- , , and are stoichiometry coefficients for gas, site, and bulk reactants.
- , , and are stoichiometry coefficients for gas, site, and bulk products.
The rate of the th reaction is then:
where and are rate exponents for reactants, and represents molar concentration on the wall. The forward rate constant for reaction is given by an Arrhenius expression. Which, for the simplest type of the reaction, is given by Eqn. (3365) (where is ).
The sum runs over all different site types.
It is assumed that the reaction rate does not depend on the concentrations of the bulk species.
The net molar rate of change per surface area (production or consumption) of each species is given by:
For some surface reactions, the reaction rate is influenced by the surface coverage characteristics of the species that are involved. The arrhenius rate expression, Eqn. (3365), can be modified to create a corrected rate constant as follows:
- Site Coverage Correction Rate Expression
Some surface reactions are characterized by the surface site coverage of species .
(3399)where , , and are the three surface coverage parameters that you can specify for species and reaction on the reacting surface. In imported reaction mechanism files, surface coverage modification reactions are indicated by the keyword, COV. See Reaction Mechanism Formats.
- Sticking Coefficient Correction Rate
Expression
Forward surface reaction rates can be expressed using sticking coefficients or sticking probabilities instead of reaction rate coefficients. Sticking coefficients describe the probability for a species to stick to a surface. The reaction can include one gas phase species and any number of surface species, the reaction rate is proportional to the coverage of all involved surface species. The sticking coefficient is calculated as:(3400)and the rate expression is modified as:(3401)
is the total surface site concentration and is the sum of all the surface reactant stoichiometric coefficients. is the molecular weight of the gas-phase species , and is the number of sites that each surface species occupies— is the reaction order for that species.
- Motz-Wise Correction Rate Expression
The rate expression for the forward reaction using the Motz-Wise correction factor is:(3402)where is calculated by Eqn. (3400).
- Bohm Correction Rate Expression
When simulating reactions which involve positive ions, you can modify the rate constant to include a Bohm velocity correction which considers the probability for the reaction to occur:(3403)
where is the electron temperature and is the molecular weight of the positive ion.
- Langmuir-Hinshelwood
Langmuir-Hinshelwood are global surface reactions that incorporate species adsorption, surface reaction and desorption into a single step. The Langmuir-Hinshelwood global rate expression applies to cases in which adsorption and desorption are assumed to be in equilibrium, and a reaction on the surface between adsorbed species is rate determining. It is assumed that the adsorption sites on the surface are independent from each other (single site adsorption), the sites are equivalent, and the surface coverage decreases the number of sites that are available for adsorption only, but does not alter the energetics of adsorption/desorption.
The effects of surface-sites being blocked by various species are included via the adsorption/desorption equilibria.The rate of progress is given by:(3408)where is the rate constant, given by Eqn. (3365), is the concentration of gas-phase species , and is the equilibrium constant for the adsorption/desorption steps, given by:(3409)The superscript exponent , corresponds to Langmuir-Hinshelwood reactions when , and to Eley-Rideal reactions when .
Particle Chemistry
For reactions of particles, the rate constant is determined using the Arrhenius equation Eqn. (3365). Since particle chemistry reaction rates consider diffusion alongside kinetics, these calculations are described in the Particle Reactions section of the Theory Guide.