Basic Definitions
Some of the specific terms that are used throughout the reacting flow theory content are summarized here.
Basic Definitions
- Cell Residence Time
- The cell residence time is the time step over which chemistry in a cell is evaluated. For unsteady flows this is the specified time-step, and for steady flows, this is the time that a fluid element spends in the cell—calculated as the mass in the cell divided by the mass flux in the cell.(3328)
- Combustion Progress Variable
-
- Unnormalized Progress Variable
- The unnormalized progress variable is used to track the progress of combustion. It is calculated by Simcenter STAR-CCM+ based either on chemical enthalpy or species weights , depending on the option that is chosen when generating the flamelet table. For species weights, is defined as:
- Unnormalized Progress Variable Variance
- The unnormalized progress variable variance is calculated using either a transport equation or by an algebraic relationship.
- Progress Variable
- The progress variable is denoted as . When , combustion has not yet commenced. When , combustion is complete. Simcenter STAR-CCM+ calculates the progress variable by considering the proportions of products that are formed, such as and during hydrocarbon combustion, or during hydrogen combustion.
- Progress Variable Variance
- The progress variable variance is defined
as:(3332)
- Equivalence Ratio
- The equivalence ratio relates the mass fraction of fuel to oxidizer in an unburned mixture to that of a stoichiometric mixture. It is calculated from the amount of carbon, hydrogen and oxygen in the mixture:(3333)(3334)(3335)
- Flamelet
- A flamelet refers to a thin laminar flame which can also be referred to in combustion simulations as a fine structure.
- Mass Fraction
- The mass fraction
of species
is given by the fraction of the total mass of all molecules of that species in a mixture
to the total mass of all molecules of all species in the mixture,
:(3336)
- Mean Molecular Weight
- The mean molecular weight of a
mixture is defined as:(3339)Combining Eqn. (3342), Eqn. (3344), Eqn. (3336), and Eqn. (3339) gives an expression for the relationship between mole and mass fractions.(3340)
- Mixture Fraction
- The mixture fraction is defined as the elemental composition that originated from the fuel stream. For example, consider a hydrocarbon molecule CnHm burning in air. The mixture fraction is then the elemental mass fraction of C and H, regardless of the state of reaction. That is, the C and H atoms can be in unburnt fuel, CnHm, or in burnt products of H₂O and CO₂.
- Mole Fraction
- The mole fraction
of species
, is given by the fraction of moles which that species occupies in the mixture
to the total number of moles of all species in the mixture,
:(3342)
- Molecular Weight
-
The molecular weight of species is given by the sum of the atomic weights of all elements in the species, weighted with the number of atoms of each element in the given species.(3344)
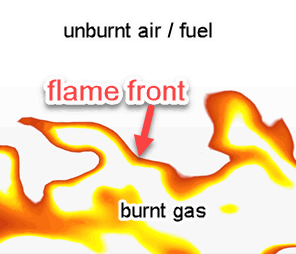
- Reaction Front (Flame Front)
- The location at which reactions occur in a flame is known as the flame front, or reaction front. To determine the position of the flame front, often the concentration of OH (an intermediate species) is monitored.
- Scalar Dissipation Rate
- The scalar dissipation rate, , is the rate at which fluctuations in scalar variables (that are caused by turbulence) are dissipated. The scalar dissipation rate is given by: (3346)
- Stoichiometric Coefficient
-
is the stoichiometric coefficient of species which defines how many moles of the species take part in reaction . For example, for a system of two reactions,
(3347)(3348)the stoichiometric coefficients are:
Species Reaction 1 Reaction 2 1 0 2 1 0 1 1 1 2 0 In the Simcenter STAR-CCM+ GUI, all stoichiometric coefficients are entered as positive numbers.
For example, reaction 1 (above) shows that for every one mole of that is consumed, there are two moles of produced.