Using the Chapman-Enskog Method for Dynamic Viscosity
This method becomes available for single- and multi-component gases once an energy model is included. This method is also activated when the Mathur-Saxena Averaging method is selected for dynamic viscosity.
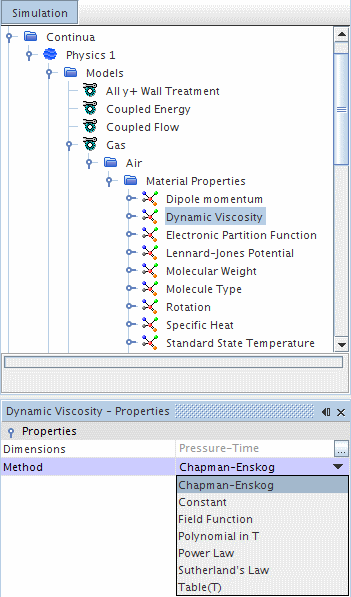
The dynamic viscosity of a simple gas, or of an individual component of a multi-component gas, is calculated with the Chapman-Enskog equation as follows:
(145)
where:
- is reduced temperature (dimensionless)
- is the Boltzmann constant
- is the potential energy of attraction of component ; values given in the standard material database, props.mdb, provided as a part of STAR-CCM+
- is the collision integral
- is the viscosity of component
- is the molecular weight of component
- is temperature (in K)
- is the collision diameter of component ; values given in the standard material database, props.mdb, provided as a part of STAR-CCM+
For a simple gas, there is only one value of .