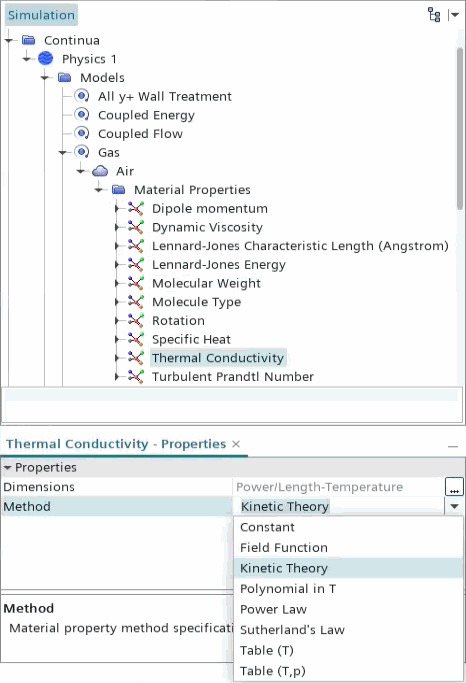
Using the Kinetic Theory Method for Thermal Conductivity
The Kinetic Theory method calculates the thermal conductivity of an individual gas component using kinetic theory.
The Kinetic Theory method for thermal conductivity is available for both single- and multi-component gases:
- For single-component gases, the Kinetic Theory method is available under the node.
- For multi-component gases, the Kinetic Theory method becomes available under the node for each gas component when you select the Mathur-Saxena Averaging method under the node.
When this method is used, the following properties nodes appear for each gas species:
- Dipole momentum — a measure of polarity of a covalent bond in the molecule (always given in Debye)
- Lennard-Jones characteristic length — the collision diameter (always given in Angstroms)
- Lennard-Jones energy — the potential energy of attraction (given in J/kmol)
- Rotation — rotational relaxation collision number (non-dimensional)
- Molecule Type — describes molecular structure;
can be one of the following:
- 0: atom
- 1: linear molecule
- 2: nonlinear molecule
These properties have only one Method available for their calculation: Constant. Their values are used to calculate the thermal conductivity of the individual gas components using the Kinetic Theory method.
Calculation of Thermal Conductivity using the Kinetic Theory method
The thermal conductivity of a simple gas, or of an individual component of a multi-component gas, is calculated as follows:
where:
and is the value of the rotation collision number at 298 K, defined in the transport properties of the component.
For a linear molecule:
- is the thermal conductivity of component .
- is the viscosity of component , .
- is the molecular weight of component .
- is the molar specific heat at constant volume of the component.
- is the universal gas constant.
- is the Lennard-Jones energy.
- is the Boltzmann constant m2 kg s-2 K-1.
For a simple gas, there is only one value of .